Relative Uptake, Metabolism, and β-Receptor Binding of (1R,2S)-4
Por um escritor misterioso
Last updated 23 fevereiro 2025
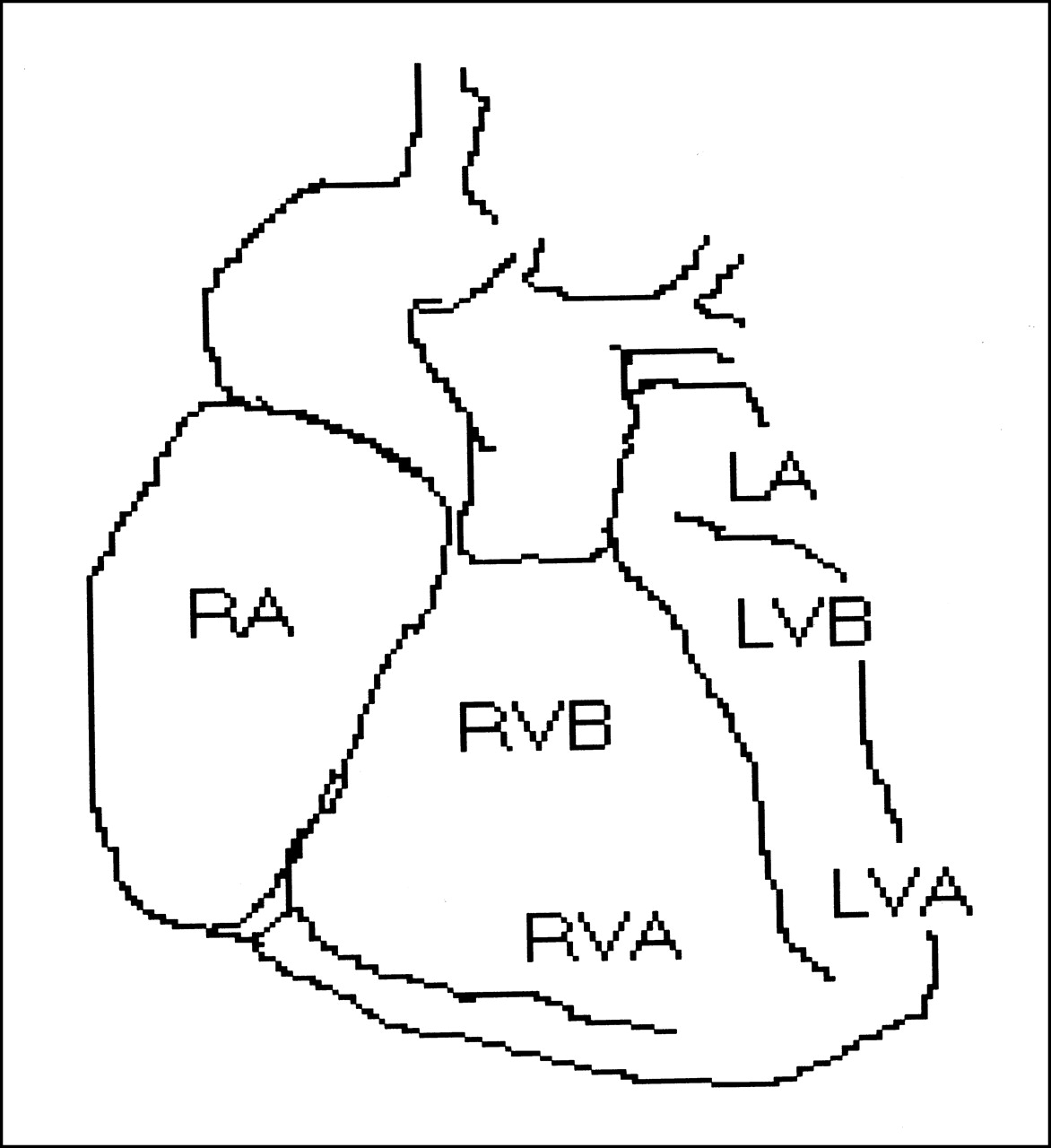
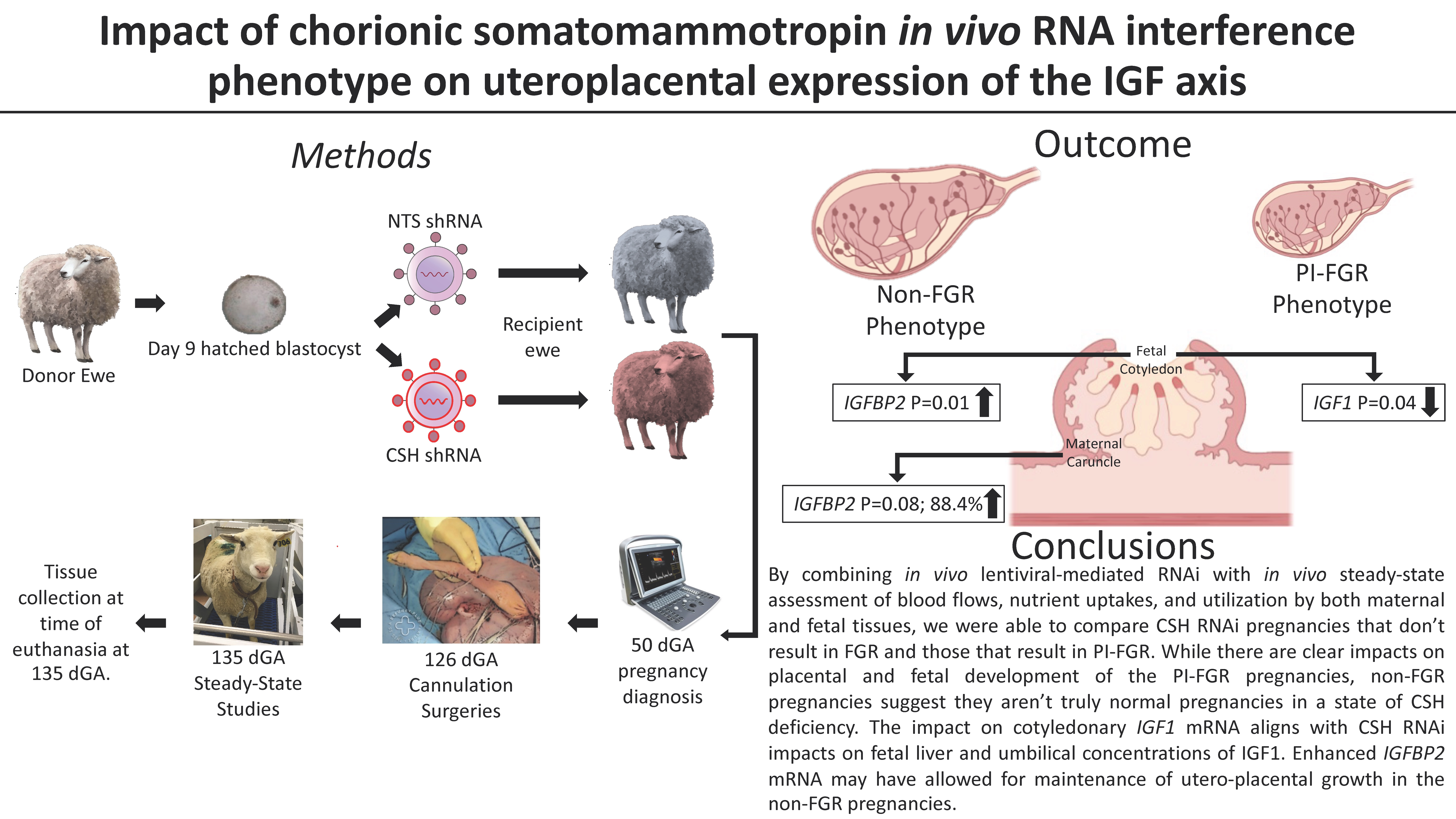
The objective of the study was to compare relative uptake, metabolism, and β-receptor affinity of the new positron-emitting uptake-1 tracer (1 R ,2 S )-4-18F-fluorometaraminol (4-FM) with those of the SPECT pharmaceutical meta-123I-iodobenzylguanidine (MIBG) in Wistar Kyoto (WKY) rats and spontaneously hypertensive (SHR) rats. Methods: No-carrier-added 4-18F-FM was applied to SHR and WKY rats in vivo and to retrogradely perfused hearts in vitro. Cardiac and extracardiac distribution was assessed, and metabolite formation was determined by thin-layer chromatography. The in vivo experiments were repeated with no-carrier-added 123I-MIBG. By means of autoradiography, the β-receptor affinity of 4-FM was compared with that of MIBG and propranolol (10 μmol/L) through displacement of 125I-iodocyanopindolol (1.5 pmol/L) in slices of heart and spleen. Results: Cardiomyopathic hearts showed heterogeneous 4-18F-FM uptake with gradients up to 3.6 in vivo and in vitro between different regions of the heart. Control hearts showed such gradients in 4-18F-FM uptake only in vitro. 123I-MIBG exhibited a less heterogeneous in vivo distribution in SHR hearts. Extracardiac differences between WKY and SHR were found for uptake of 4-18F-FM in the spleen (63.3% ± 4% vs. 38.8% ± 5.7% of cardiac activity) and for renal uptake of 123I-MIBG (373% ± 27% vs. 81.4% ± 17% of cardiac activity). Metabolites of 4-18F-FM were found only in the liver and those of 123I-MIBG were found in the liver and kidney with a nearly equal relative fraction in both types of animals of about 20%, 60%, and 30%, respectively. 4-FM suppressed cardiac-specific β-receptor binding of 125I-iodocyanopindolol in heart and spleen of both types of animals significantly, whereas MIBG had almost no effect. Conclusion: The more heterogeneous cardiac distribution of 4-18F-FM suggests that it reflects alterations in uptake-1 better than 123I-MIBG in addition to the possibility of quantification and higher spatial resolution by PET compared with SPECT. Altered biotransformation in cardiomyopathic diseases may also impair the evaluation of 123I-MIBG-SPECT data. The β-receptor binding of 4-18F-FM must be further elucidated.

The beta2‐adrenergic receptor – a re‐emerging target to combat obesity and induce leanness? - Hostrup - 2022 - The Journal of Physiology - Wiley Online Library

Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease - ScienceDirect

Calciprotein Particles Induce Endothelial Dysfunction by Impairing Endothelial Nitric Oxide Metabolism

IJMS, Free Full-Text
IJMS, Free Full-Text

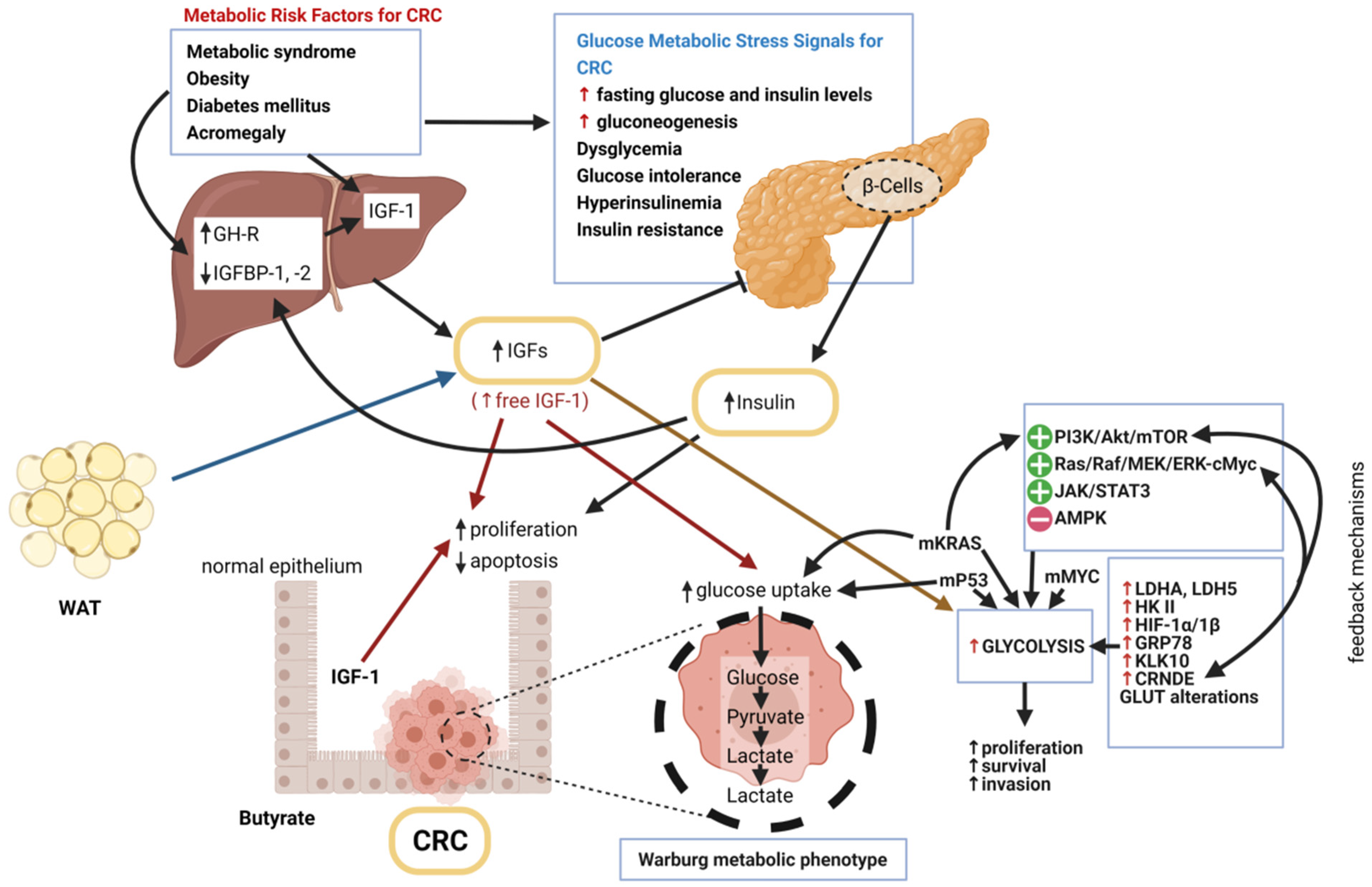
Mitochondria and T2D: Role of Autophagy, ER Stress, and Inflammasome: Trends in Endocrinology & Metabolism

Microbial-host-isozyme analyses reveal microbial DPP4 as a potential antidiabetic target
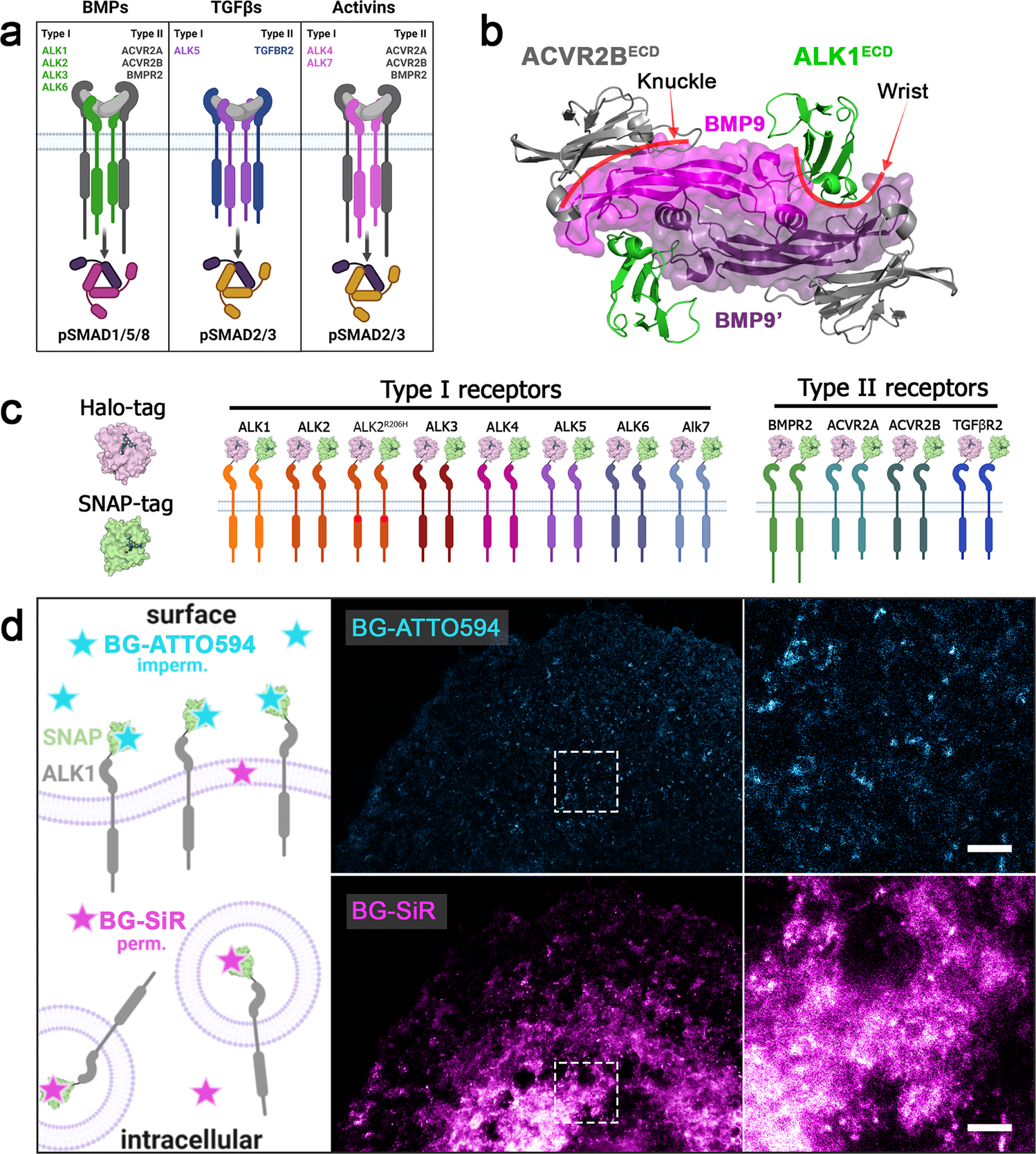
A versatile Halo- and SNAP-tagged BMP/TGFβ receptor library for quantification of cell surface ligand binding

Chimeric TIM-4 receptor-modified T cells targeting phosphatidylserine mediates both cytotoxic anti-tumor responses and phagocytic uptake of tumor-associated antigen for T cell cross-presentation: Molecular Therapy
Vitamin D receptor (VDR) action at target cells. Intracellular
Recomendado para você
-
 Radio Venancio Aires - AM 910 - Venancio Aires, RS - Ouça Online23 fevereiro 2025
Radio Venancio Aires - AM 910 - Venancio Aires, RS - Ouça Online23 fevereiro 2025 -
The Sanctuary Colonial Heights VA23 fevereiro 2025
-
 Cans & Paddles Set Qty 20023 fevereiro 2025
Cans & Paddles Set Qty 20023 fevereiro 2025 -
 Ezequiel 34:8 RVA Mobile Phone Wallpaper - Vivo yo, ha dicho el23 fevereiro 2025
Ezequiel 34:8 RVA Mobile Phone Wallpaper - Vivo yo, ha dicho el23 fevereiro 2025 -
 File:ARV logo 2018.svg - Wikimedia Commons23 fevereiro 2025
File:ARV logo 2018.svg - Wikimedia Commons23 fevereiro 2025 -
Un Café con el señor Mayor General (Rva) MANUEL GUZMÁN CARDOZO23 fevereiro 2025
-
 Life, Free Full-Text23 fevereiro 2025
Life, Free Full-Text23 fevereiro 2025 -
 Vivo Living Baltimore, Baltimore, MD23 fevereiro 2025
Vivo Living Baltimore, Baltimore, MD23 fevereiro 2025 -
 The structure, properties and potential probiotic properties of starch-pectin blend: A review - ScienceDirect23 fevereiro 2025
The structure, properties and potential probiotic properties of starch-pectin blend: A review - ScienceDirect23 fevereiro 2025 -
 RVA em direto Rádio Online Grátis23 fevereiro 2025
RVA em direto Rádio Online Grátis23 fevereiro 2025
você pode gostar
-
 Imprimir quebra cabeça O Comboio dos Dinossauros 3 O comboio dos dinossauros, Artesanato de dinossauro, Dinossauros para impressão23 fevereiro 2025
Imprimir quebra cabeça O Comboio dos Dinossauros 3 O comboio dos dinossauros, Artesanato de dinossauro, Dinossauros para impressão23 fevereiro 2025 -
 Top 5 Clicker Games! - or: Top 5 Idle Games! (Best Clicker Games23 fevereiro 2025
Top 5 Clicker Games! - or: Top 5 Idle Games! (Best Clicker Games23 fevereiro 2025 -
 Kyokou Suiri Ep. 2: A neat story will do23 fevereiro 2025
Kyokou Suiri Ep. 2: A neat story will do23 fevereiro 2025 -
 Hillary Clinton says she'll support Sanders if he's nominated by23 fevereiro 2025
Hillary Clinton says she'll support Sanders if he's nominated by23 fevereiro 2025 -
 Jogo Educativo de Matemática e Pedagógico Joga Joga Tabuada23 fevereiro 2025
Jogo Educativo de Matemática e Pedagógico Joga Joga Tabuada23 fevereiro 2025 -
 Japan : r/CountryHumans23 fevereiro 2025
Japan : r/CountryHumans23 fevereiro 2025 -
![AmiAmi [Character & Hobby Shop] [Bonus] DVD Movie PreCure All Stars F Regular Edition(Pre-order)](https://img.amiami.com/images/product/thumb300/234/MED-DVD2-55395.jpg) AmiAmi [Character & Hobby Shop] [Bonus] DVD Movie PreCure All Stars F Regular Edition(Pre-order)23 fevereiro 2025
AmiAmi [Character & Hobby Shop] [Bonus] DVD Movie PreCure All Stars F Regular Edition(Pre-order)23 fevereiro 2025 -
 Chani on X: Picture from One Piece chapter 1044 colored #ONEPIECE1044 # ONEPIECE #ONEPIECESPOILERS / X23 fevereiro 2025
Chani on X: Picture from One Piece chapter 1044 colored #ONEPIECE1044 # ONEPIECE #ONEPIECESPOILERS / X23 fevereiro 2025 -
 Jogos de Play no Jogos 36023 fevereiro 2025
Jogos de Play no Jogos 36023 fevereiro 2025 -
 1 conjunto rosa vestido de festa roupas para barbie boneca puff manga saia magro vestido feito à mão roupa para 1/6 fr dollhoues brinquedos - AliExpress23 fevereiro 2025
1 conjunto rosa vestido de festa roupas para barbie boneca puff manga saia magro vestido feito à mão roupa para 1/6 fr dollhoues brinquedos - AliExpress23 fevereiro 2025

