Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Por um escritor misterioso
Last updated 27 dezembro 2024
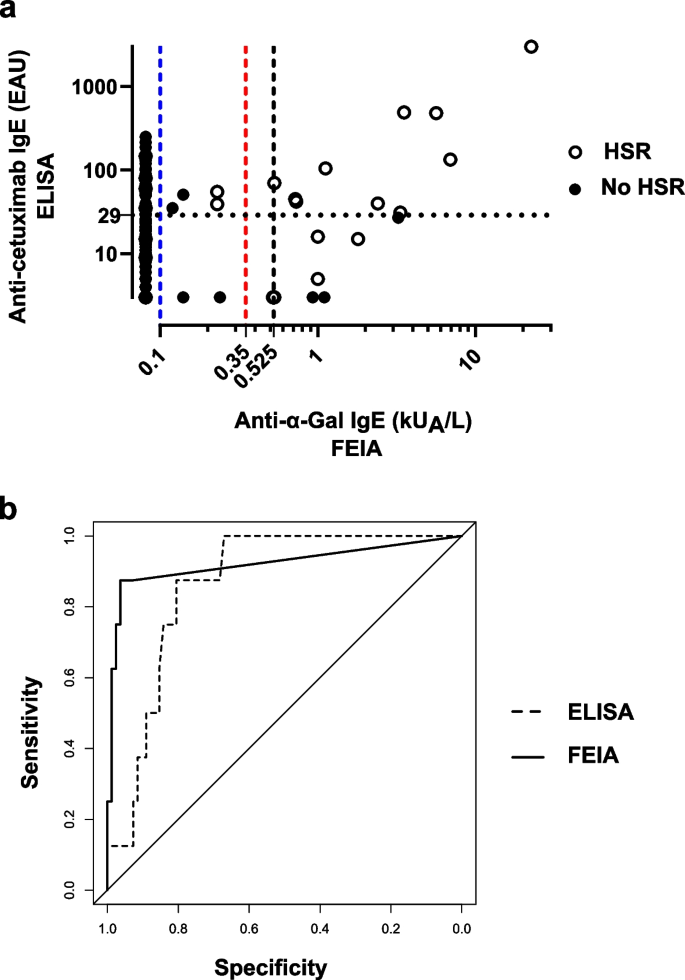
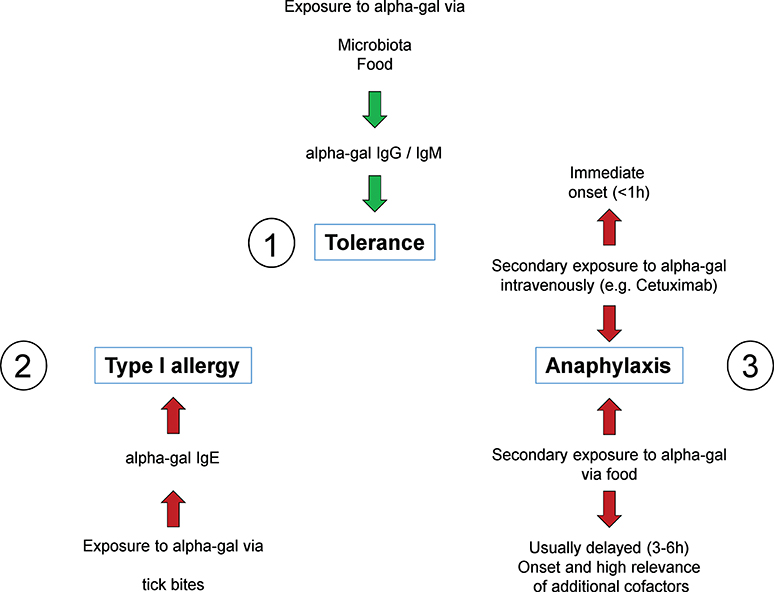
Background The link between immediate hypersensitivity reactions (HSR) following the first cetuximab infusion and the IgE sensitization against anti-galactose-α-1,3-galactose (α-Gal) is now well-established. An automated Fluoroenzyme-Immunoassay (FEIA) is available and may facilitate the screening of patients with anti-α-Gal IgE before treatment. Methods This study aimed to evaluate its performances as compared to a previously validated anti-cetuximab IgE ELISA, using 185 samples from two previously studied cohorts. Results Despite 21.1% of discrepancies between the two techniques, FEIA discriminated better positive patients and similarly negative ones with a ≥ 0.525 kUA/L threshold. Sensitivity was 87.5% for both tests, specificity was better for FEIA (96.3% vs ELISA: 82.1%). FEIA had a higher positive likelihood ratio (23.9 vs ELISA: 4.89) and a similar negative likelihood ratio (0.13 vs ELISA: 0.15). In our population, the risk of severe HSR following a positive test was higher with FEIA (56.7% vs ELISA: 19.6%) and similar following a negative test (0.7% vs ELISA: 0.8%). Conclusion Although the predictive value of the IgE screening before cetuximab infusion remains discussed, this automated commercial test can identify high-risk patients and is suitable for routine use in laboratories. It could help avoiding cetuximab-induced HSR by a systematic anti-α-Gal IgE screening before treatment.

Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer

PDF) A retrospective analysis of cross-reacting cetuximab IgE antibody and its association with severe infusion reactions

Description of the study population. HSR: Hypersensitivity reaction.
Frontiers The History of Carbohydrates in Type I Allergy

Forest plot and SROC curve. Download Scientific Diagram

(PDF) Case Report About Fatal or Near-Fatal Hypersensitivity Reactions to Cetuximab: Anticetuximab IgE as a Valuable Screening Test

Manifestations of Antidrug Antibodies Response: Hypersensitivity and Infusion Reactions

Nationwide pharmacovigilance data for cetuximab-induced anaphylaxis and predictive model validation using prospective specific IgE detection - ScienceDirect

The role of IgE specific for galactose-α-1,3-galactose in predicting cetuximab induced hypersensitivity reaction: a systematic review and a diagnostic meta-analysis

Full article: Current and Future Strategies for the Diagnosis and Treatment of the Alpha-Gal Syndrome (AGS)

The future of food allergy: Challenging existing paradigms of clinical practice - Anagnostou - 2023 - Allergy - Wiley Online Library

Description of the study population. HSR: Hypersensitivity reaction.
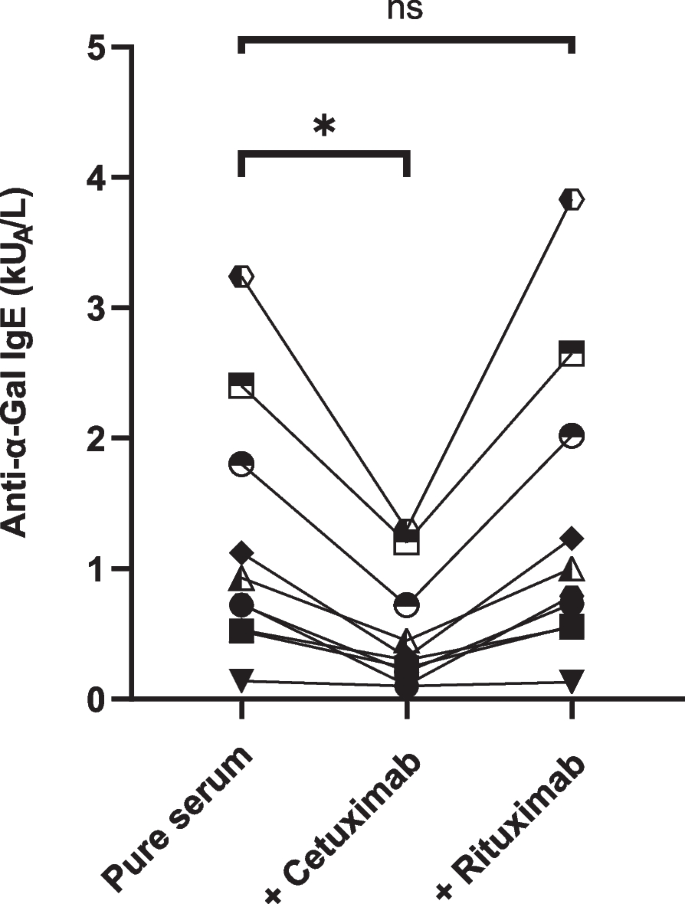
Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
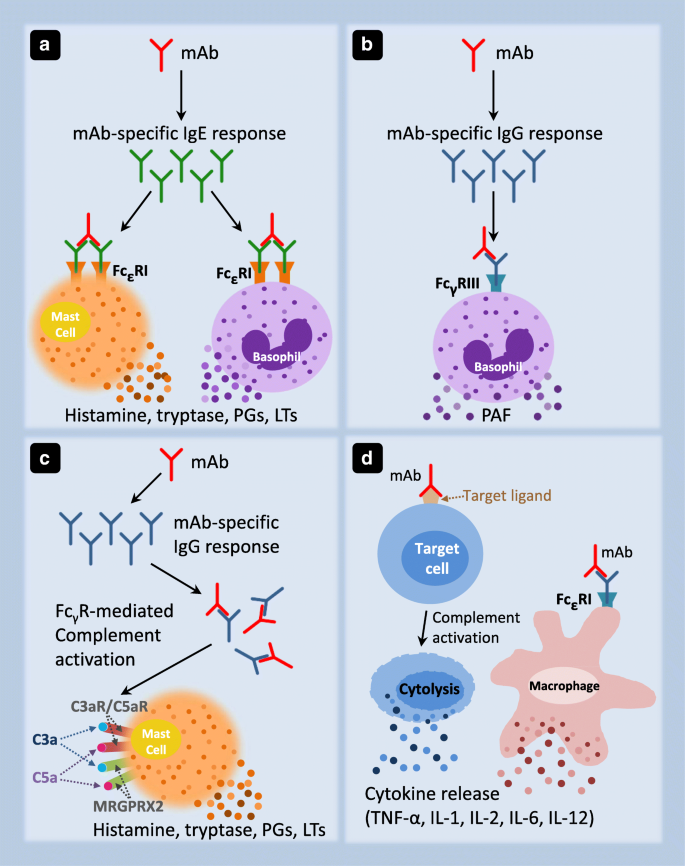
Anaphylaxis Induced by Biologics Current Treatment Options in Allergy

Validation of an anti-α-Gal IgE fluoroenzyme-immunoassay for the screening of patients at risk of severe anaphylaxis to cetuximab, BMC Cancer
Recomendado para você
-
Disney Frozen Free Fall – Apps no Google Play27 dezembro 2024
-
 Video viral: Esta fue la reacción de una niña al ver su pastel mal hecho de 'Frozen27 dezembro 2024
Video viral: Esta fue la reacción de una niña al ver su pastel mal hecho de 'Frozen27 dezembro 2024 -
 La reacción viral de una niña tras recibir una torta fallida de la película Frozen27 dezembro 2024
La reacción viral de una niña tras recibir una torta fallida de la película Frozen27 dezembro 2024 -
 TITANS The Queen and the Beast (Godzilla and Elsa) by Dark-Rider28 on DeviantArt27 dezembro 2024
TITANS The Queen and the Beast (Godzilla and Elsa) by Dark-Rider28 on DeviantArt27 dezembro 2024 -
 Memetizando – Página: Array – Acabando com a sua produtividade – Blog de Humor – Tirinhas – Gifs – Prints Engraçados – Videos engraçados e memes do Brasil.27 dezembro 2024
Memetizando – Página: Array – Acabando com a sua produtividade – Blog de Humor – Tirinhas – Gifs – Prints Engraçados – Videos engraçados e memes do Brasil.27 dezembro 2024 -
 Seamens strike hi-res stock photography and images - Alamy27 dezembro 2024
Seamens strike hi-res stock photography and images - Alamy27 dezembro 2024 -
 Festa Frozen: passo a passo e 85 ideias encantadoras27 dezembro 2024
Festa Frozen: passo a passo e 85 ideias encantadoras27 dezembro 2024 -
 Foto: Vanessa Hudgens ainda pode ser vista em 'Manchete Kills', 'The Frozen Ground', e em 'Spring Breakers', com Selena Gomez - Purepeople27 dezembro 2024
Foto: Vanessa Hudgens ainda pode ser vista em 'Manchete Kills', 'The Frozen Ground', e em 'Spring Breakers', com Selena Gomez - Purepeople27 dezembro 2024 -
 Saiu o trailer de Ghostbusters: Apocalipse de Gelo27 dezembro 2024
Saiu o trailer de Ghostbusters: Apocalipse de Gelo27 dezembro 2024 -
 Stella, A Feia (Portuguese Edition) by K.M. Mendes27 dezembro 2024
Stella, A Feia (Portuguese Edition) by K.M. Mendes27 dezembro 2024
você pode gostar
-
 Tensei Shitara Slime Datta Ken (OVA)27 dezembro 2024
Tensei Shitara Slime Datta Ken (OVA)27 dezembro 2024 -
 Analista prevê que a venda da Warner Bros Games irá desencadear uma onda de Fusões e Mega-Aquisições27 dezembro 2024
Analista prevê que a venda da Warner Bros Games irá desencadear uma onda de Fusões e Mega-Aquisições27 dezembro 2024 -
 The Marginal Service: A Talking Squirrel, Fit Bodies, & Aliens27 dezembro 2024
The Marginal Service: A Talking Squirrel, Fit Bodies, & Aliens27 dezembro 2024 -
 giria-mar-abr-201227 dezembro 2024
giria-mar-abr-201227 dezembro 2024 -
 Genshin Impact 3.1 Códigos Setembro 2022: Primogems gratuitos e como redimi-los27 dezembro 2024
Genshin Impact 3.1 Códigos Setembro 2022: Primogems gratuitos e como redimi-los27 dezembro 2024 -
 Ultra Sun and Moon Pokedex Entries27 dezembro 2024
Ultra Sun and Moon Pokedex Entries27 dezembro 2024 -
FStik: All Telegram Stickers for Android - Free App Download27 dezembro 2024
-
 I was wondering if you can mod like Clothes in gta 5 for the Xbox 360 I have a rgh so it's not usb mods lol. I think it might be able27 dezembro 2024
I was wondering if you can mod like Clothes in gta 5 for the Xbox 360 I have a rgh so it's not usb mods lol. I think it might be able27 dezembro 2024 -
 jogo papagaio madeira, poleiros treinamento do papagaio, Poleiros madeira portáteis e reutilizáveis para brincar e viajar com pássaros, poleiros27 dezembro 2024
jogo papagaio madeira, poleiros treinamento do papagaio, Poleiros madeira portáteis e reutilizáveis para brincar e viajar com pássaros, poleiros27 dezembro 2024 -
 brasil e argentina countryhumans27 dezembro 2024
brasil e argentina countryhumans27 dezembro 2024