FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Last updated 27 dezembro 2024

Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

World's First Gene Therapy Clinical Trial for Glycogen Storage Disease Approved by FDA - Connecticut Children's
Department of Labor and Food and Drug Administration Confirmations Hearing

MUSINGS OF A CANCER DOCTOR: War, Malaria, and the Nobel Prize, Article

Kentucky kidney transplant patient wants trial after getting cancer
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/Druker-cancer-with-patient-631.jpg)
A Triumph in the War Against Cancer, Science
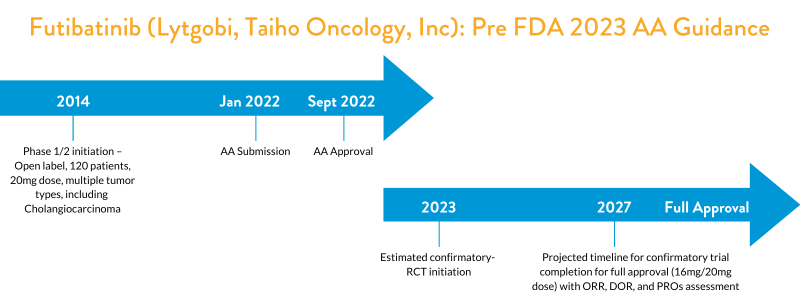
Accelerated Approval for Anticancer Products

Elias Jabbour MD Anderson Cancer Center

Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial - The Lancet Oncology

Harnessing the Immune System to Fight Cancer - The New York Times

Controversial Texas doctor Stanislaw Burzynski goes before disciplinary board
Recomendado para você
-
 About Us27 dezembro 2024
About Us27 dezembro 2024 -
 Dr. Caron Houston Makes House Calls - Inside Sacramento27 dezembro 2024
Dr. Caron Houston Makes House Calls - Inside Sacramento27 dezembro 2024 -
🚨New🚨🩺Dr. Joye Taylor-Houston Haematologist / Oncologist : Specializing in: ▪️Benign Haematology Anaemia Thrombocytopenia…27 dezembro 2024
-
 Houston Methodist The Woodlands now offers two Transcatheter Aortic Valve Replacement Options - Hello Woodlands27 dezembro 2024
Houston Methodist The Woodlands now offers two Transcatheter Aortic Valve Replacement Options - Hello Woodlands27 dezembro 2024 -
 Is My 600-Lb Life's Dr. Now a real doctor? Where is he from?27 dezembro 2024
Is My 600-Lb Life's Dr. Now a real doctor? Where is he from?27 dezembro 2024 -
 Sarasota cancer survivor pays it forward, with blanket warmers for patients27 dezembro 2024
Sarasota cancer survivor pays it forward, with blanket warmers for patients27 dezembro 2024 -
Kassandra Alfaro27 dezembro 2024
-
 After-Hours Care — Rockin' Pets, Rollin' Vets27 dezembro 2024
After-Hours Care — Rockin' Pets, Rollin' Vets27 dezembro 2024 -
 Weightless Excerpt: My 600-lb Life Needs To Be Canceled27 dezembro 2024
Weightless Excerpt: My 600-lb Life Needs To Be Canceled27 dezembro 2024 -
 Does Dr. Now Have a Wife? What to Know About the Doctor From 'My 600-lb Life27 dezembro 2024
Does Dr. Now Have a Wife? What to Know About the Doctor From 'My 600-lb Life27 dezembro 2024
você pode gostar
-
 DESAFIO: Você consegue acertar em qual país nasceram esses 2527 dezembro 2024
DESAFIO: Você consegue acertar em qual país nasceram esses 2527 dezembro 2024 -
 Pai, Mãe, Eu Nós entendemos, Itachi, Itachi, Apenas prometa isso Cuide do Sasuke - iFunny Brazil27 dezembro 2024
Pai, Mãe, Eu Nós entendemos, Itachi, Itachi, Apenas prometa isso Cuide do Sasuke - iFunny Brazil27 dezembro 2024 -
 Cristiano Ronaldo Awful Foul???🔥⚽🤯27 dezembro 2024
Cristiano Ronaldo Awful Foul???🔥⚽🤯27 dezembro 2024 -
Atelier Ryza 2: Lost Legends & The Secret Fairy PS4 Save Wizard Cheat Codes & Resources27 dezembro 2024
-
 3D Kanojo Iroha and Hikari reunited27 dezembro 2024
3D Kanojo Iroha and Hikari reunited27 dezembro 2024 -
 WDL Inventory and Operative Editor at Watch Dogs: Legion Nexus27 dezembro 2024
WDL Inventory and Operative Editor at Watch Dogs: Legion Nexus27 dezembro 2024 -
 Bigg Boss Season 13: Salman Khan's Show To Feature 2 Teams27 dezembro 2024
Bigg Boss Season 13: Salman Khan's Show To Feature 2 Teams27 dezembro 2024 -
 Mini Moto Trilha Cross Off Road Laminha 100cc 4 Tempos27 dezembro 2024
Mini Moto Trilha Cross Off Road Laminha 100cc 4 Tempos27 dezembro 2024 -
 Cabeleireiro Fêmea Que Faz O Corte De Cabelo Masculino Com a Lâmina Elétrica Do Cabelo No Salão De Beleza Do Cabeleireiro No Ar L Video Estoque - Vídeo de aparamento, beleza: 11828443927 dezembro 2024
Cabeleireiro Fêmea Que Faz O Corte De Cabelo Masculino Com a Lâmina Elétrica Do Cabelo No Salão De Beleza Do Cabeleireiro No Ar L Video Estoque - Vídeo de aparamento, beleza: 11828443927 dezembro 2024 -
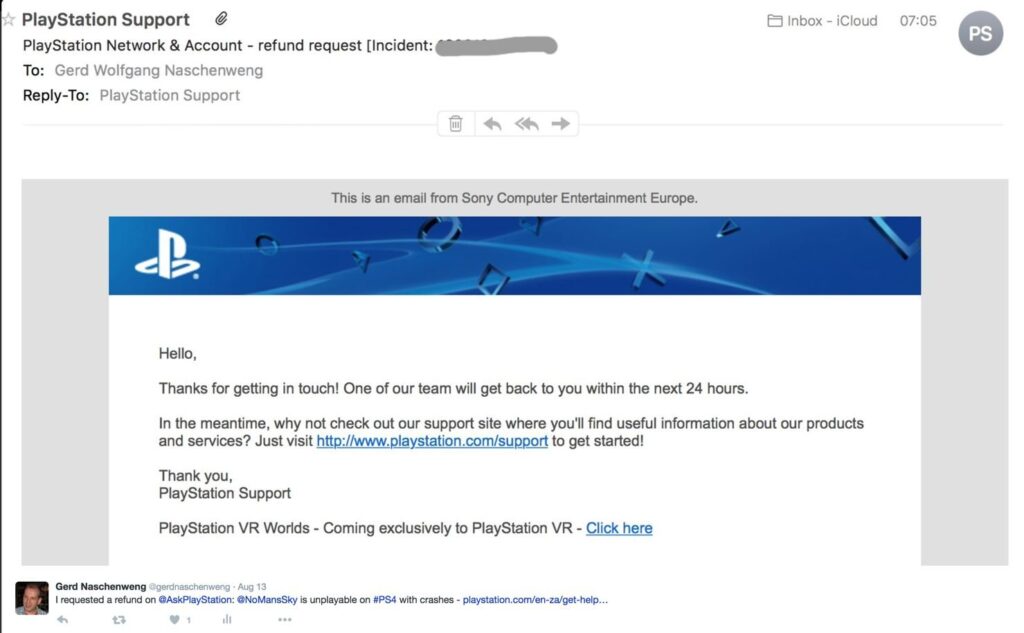 Requesting a refund for No Man's Sky will get your PSN account banned27 dezembro 2024
Requesting a refund for No Man's Sky will get your PSN account banned27 dezembro 2024

