FDA won't comment on status of Emergency Use Authorizations for two antibody treatments
Por um escritor misterioso
Last updated 04 março 2025

The US Food and Drug Administration told CNN Thursday morning that the agency doesn’t have any comments on the applications for Emergency Use Authorizations for Eli Lilly and Regeneron antibody treatments.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
The coronavirus pandemic has brought countries to a standstill. In many places, as countries reopen, Covid-19 cases are on the rise. Follow here for the latest.
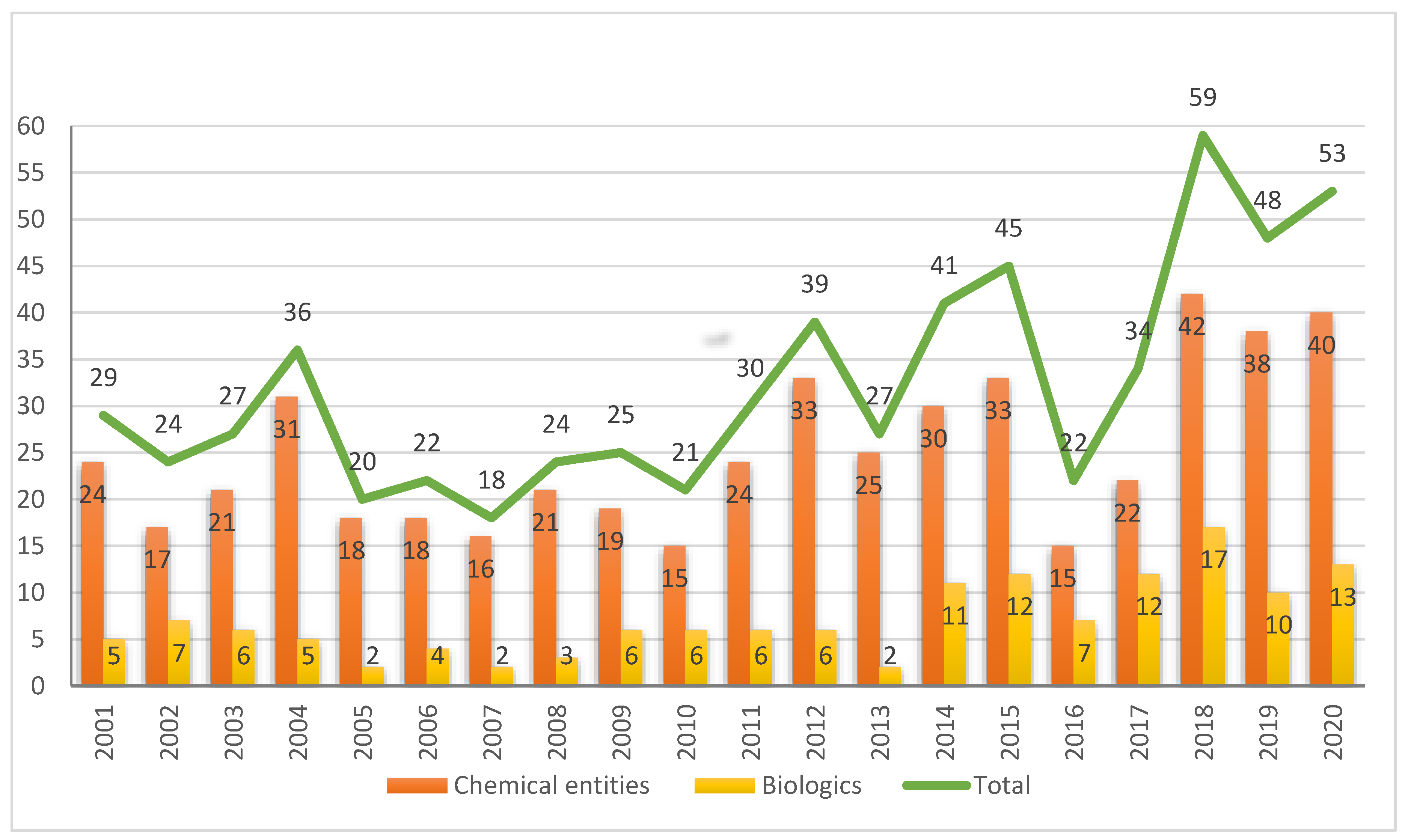
Molecules, Free Full-Text

Federal Register :: Authorizations of Emergency Use of Certain

Covid-19: F.D.A. Panel Gives Green Light to Johnson & Johnson's

FDA Revokes Emergency Use Authorization for Monoclonal
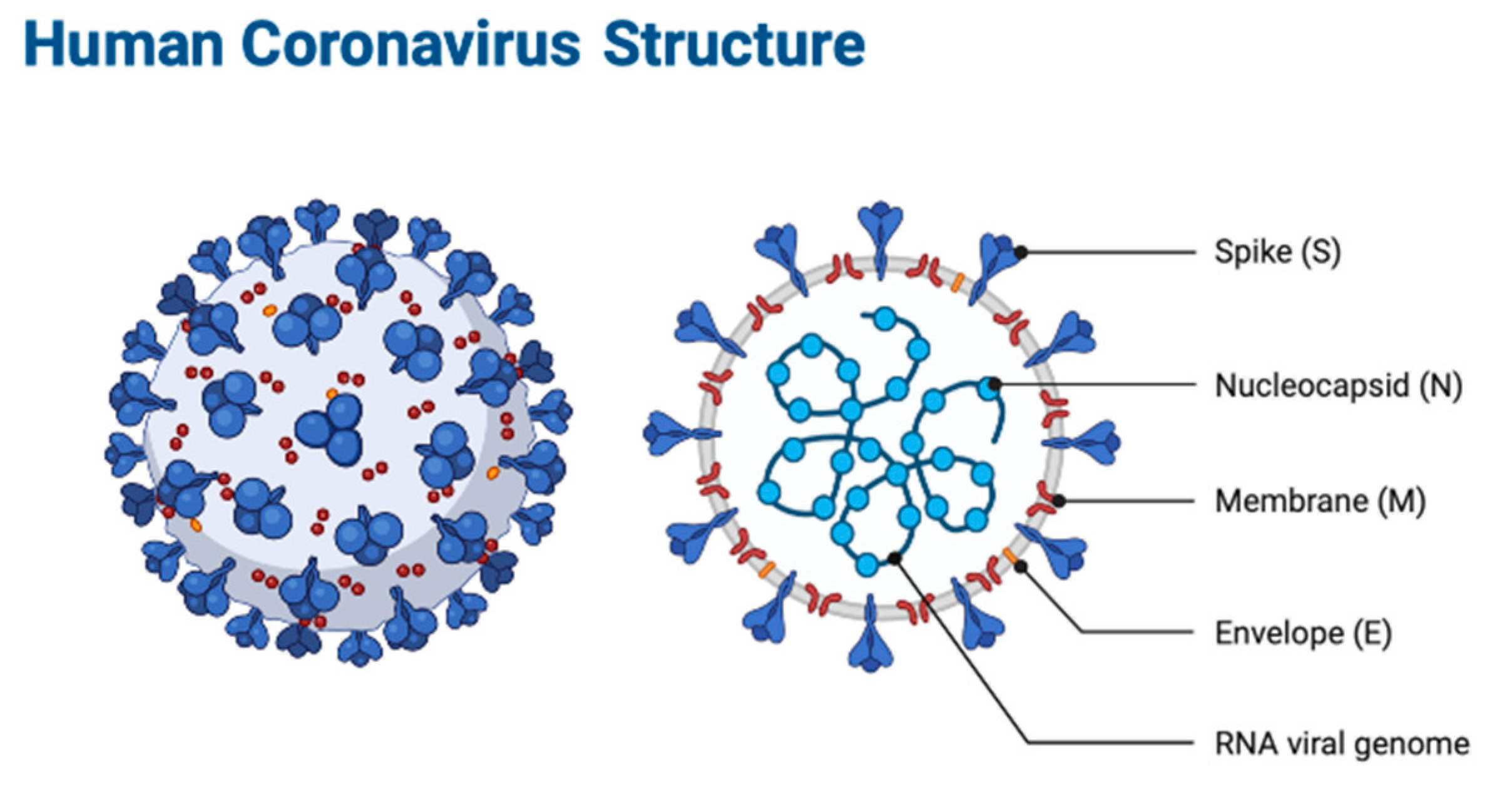
IJMS, Free Full-Text

Federal Register :: Authorizations of Emergency Use of Certain
FDA Meeting on COVID Vaccine for Children

Eli Lilly's Antibody Treatment Gets Emergency F.D.A. Approval
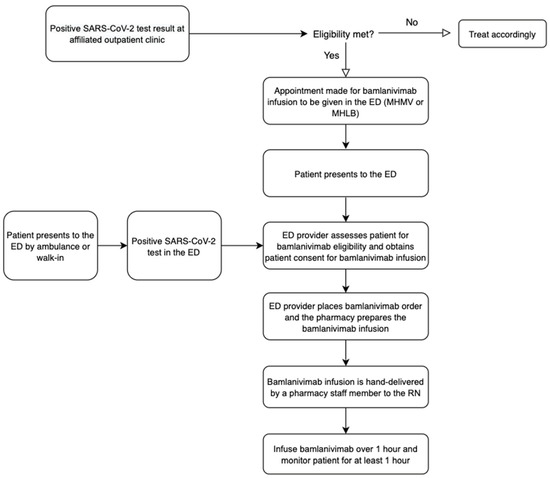
Healthcare, Free Full-Text
Recomendado para você
-
 Dr. Isaac Azar, MD – Aventura, FL04 março 2025
Dr. Isaac Azar, MD – Aventura, FL04 março 2025 -
 Emerson Sheik mima afilhado, Jean-Luc, filho de Isaac Azar04 março 2025
Emerson Sheik mima afilhado, Jean-Luc, filho de Isaac Azar04 março 2025 -
 O brit milá do caçula do chef Isaac Azar04 março 2025
O brit milá do caçula do chef Isaac Azar04 março 2025 -
 Histórias e raízes de Angelita e Isaac04 março 2025
Histórias e raízes de Angelita e Isaac04 março 2025 -
 Dr. Isaac D. Azar, MD, Aventura, FL, Emergency Medicine Physician04 março 2025
Dr. Isaac D. Azar, MD, Aventura, FL, Emergency Medicine Physician04 março 2025 -
 Dr Isaac Karimi CV04 março 2025
Dr Isaac Karimi CV04 março 2025 -
 Affiliate Faculty, Richard and Loan Hill Department of Biomedical Engineering04 março 2025
Affiliate Faculty, Richard and Loan Hill Department of Biomedical Engineering04 março 2025 -
 Johns Hopkins Defeats Franklin & Marshall and Dickinson - Johns Hopkins University Athletics04 março 2025
Johns Hopkins Defeats Franklin & Marshall and Dickinson - Johns Hopkins University Athletics04 março 2025 -
 Paris 6 restaurant hi-res stock photography and images - Page 3 - Alamy04 março 2025
Paris 6 restaurant hi-res stock photography and images - Page 3 - Alamy04 março 2025 -
 Newsletter January 2022: Editorial – Is anaesthesia mortality a source of stress for the anaesthesiologist?04 março 2025
Newsletter January 2022: Editorial – Is anaesthesia mortality a source of stress for the anaesthesiologist?04 março 2025
você pode gostar
-
 Bebe Reborn Girafinha Boneca Silicone 100% Mercado Livre Lol04 março 2025
Bebe Reborn Girafinha Boneca Silicone 100% Mercado Livre Lol04 março 2025 -
The tie breaker just broke the biggest super bowl curses04 março 2025
-
 Champions' names according to autocorrect : r/LeagueOfMemes04 março 2025
Champions' names according to autocorrect : r/LeagueOfMemes04 março 2025 -
 About TopTier Palletizers04 março 2025
About TopTier Palletizers04 março 2025 -
 HeartGold and SoulSilver, Pokédex Black And White, Pokémon Omega Ruby, Alpha Sap 978030746803104 março 2025
HeartGold and SoulSilver, Pokédex Black And White, Pokémon Omega Ruby, Alpha Sap 978030746803104 março 2025 -
 I just finished my 1,857th viewing and I noticed the name of the company is Dunder Mifflin. If you look closely you can see it on this sign. : r/DunderMifflin04 março 2025
I just finished my 1,857th viewing and I noticed the name of the company is Dunder Mifflin. If you look closely you can see it on this sign. : r/DunderMifflin04 março 2025 -
 How To Start EA FC 24 on the WEB APP! EA FC 24 Ultimate Team04 março 2025
How To Start EA FC 24 on the WEB APP! EA FC 24 Ultimate Team04 março 2025 -
 Listen to Made in Abyss Movie 3: Fukaki Tamashii no Reimei OST ORIGINAL SOUNDTRACK on Apple Music & Spotify04 março 2025
Listen to Made in Abyss Movie 3: Fukaki Tamashii no Reimei OST ORIGINAL SOUNDTRACK on Apple Music & Spotify04 março 2025 -
 Stream BoyWithUke-Toxic (Genius OpenMic Live Performance) by Johann.sue0604 março 2025
Stream BoyWithUke-Toxic (Genius OpenMic Live Performance) by Johann.sue0604 março 2025 -
 Kind of related to this. Were memes banned though?, /r/Doomers, Doomer04 março 2025
Kind of related to this. Were memes banned though?, /r/Doomers, Doomer04 março 2025
