What Does the IRB Review?, Research
Por um escritor misterioso
Last updated 06 fevereiro 2025

Below are the elements the IRB looks for when reviewing research. Federal regulations 45 CFR 46.111 and 21 CFR 56.111 outline the requirements for approval of non-exempt human subjects research. To obtain IRB approval, the IRB must have enough information to determine the criteria in each of the sections below are satisfied.
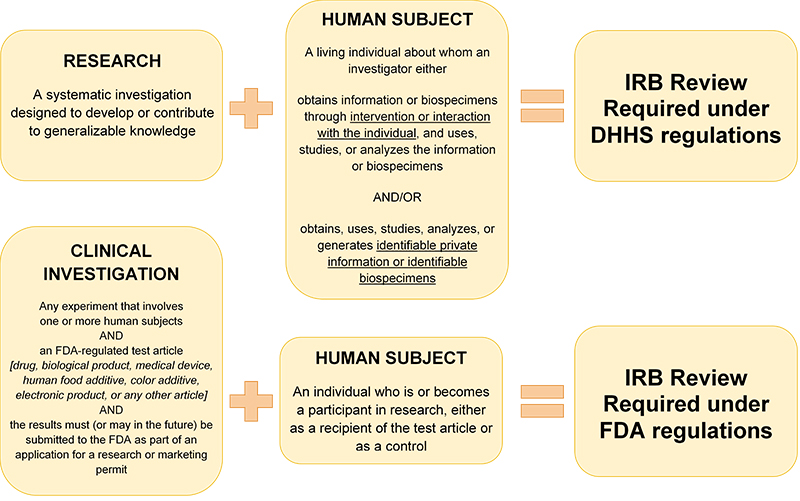
Activities requiring IRB review - Virginia Commonwealth University

IRB Review Levels and Examples

Requirements for Institutional Review Board (IRB) Review and HIPAA Waiver Documentation for RIF DUA Request Submissions

What is an IRB?
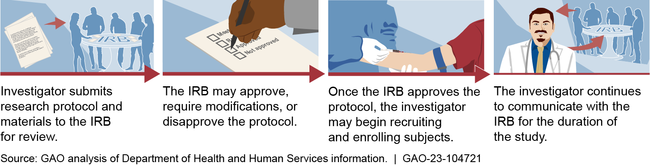
Institutional Review Boards: Actions Needed to Improve Federal Oversight and Examine Effectiveness

Human Subjects Research
Doctoral Dissertation Research and the IRB, 2021, IRB Blog, Institutional Review Board

Webinar: What You Should Know About IRB Review of Research

Frequently Asked Questions (FAQ)
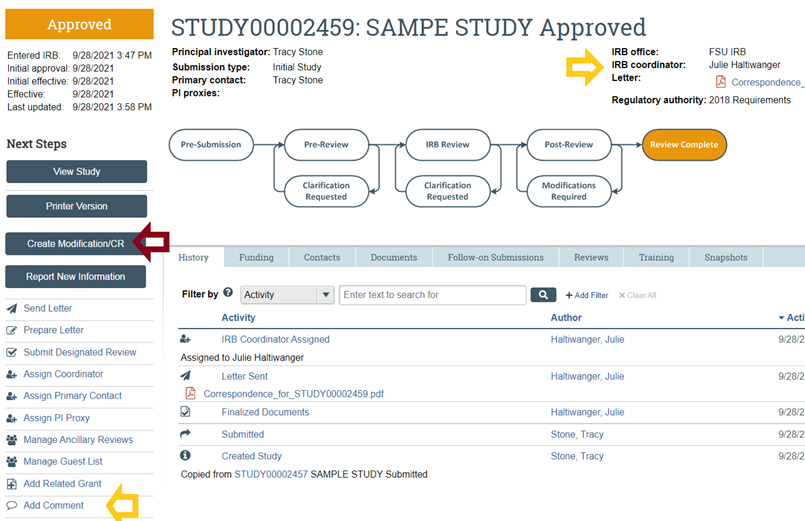
FAQs FSU Office of Research
Institutional Review Board - Barry University, Miami, FL
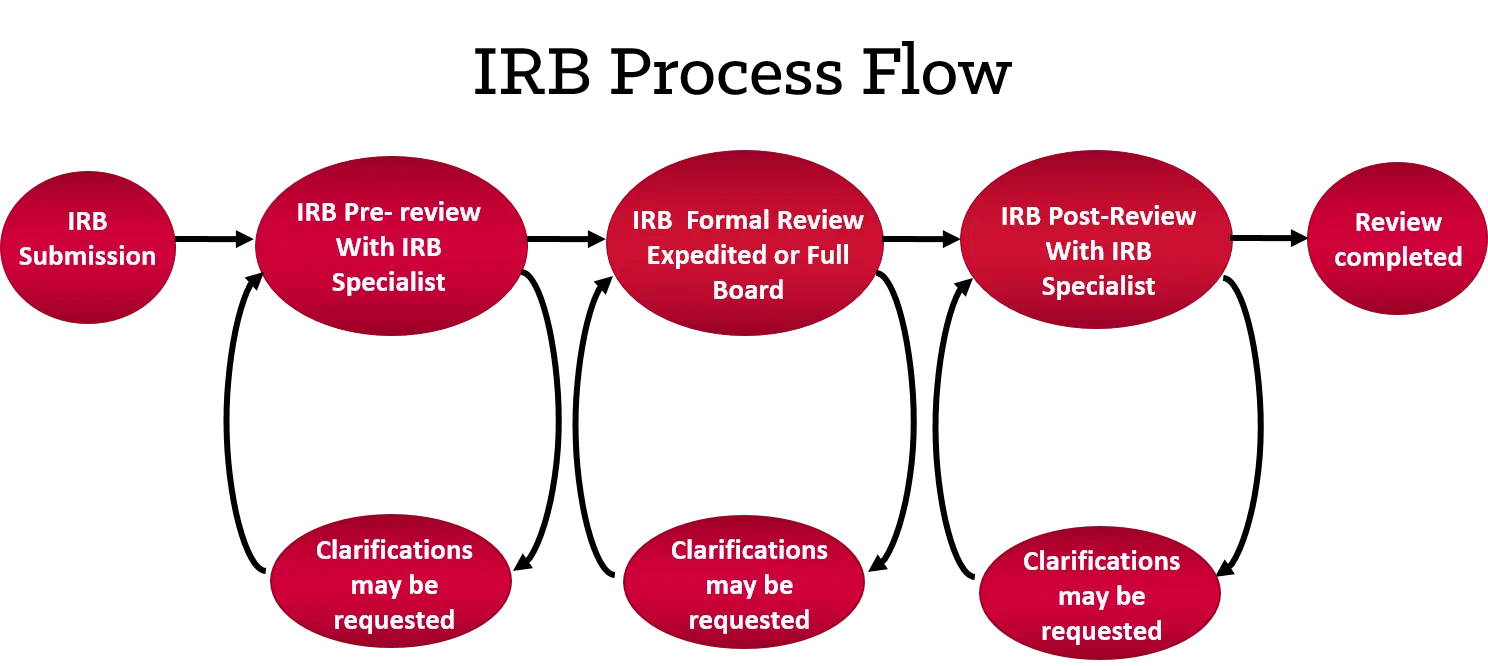
Institutional Review Board, Human Research Protection Program, University Hospitals, Cleveland, OH
Recomendado para você
-
IRBSL06 fevereiro 2025
-
IRBSL - ♥poussin♥☺Sidi Lakhdar06 fevereiro 2025
-
 QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback06 fevereiro 2025
QIDIAN Calcomanía de aleación 3D para parachoques delantero de coche, insignia para Cooper S ONE R55 R56 F54 F55 F56 F57 F60 R60 Clubman Hatchback06 fevereiro 2025 -
 Teenage Boy Flexing Muscles Stock Photo - Download Image Now - Muscular Build, Swimming Pool, Teenage Boys - iStock06 fevereiro 2025
Teenage Boy Flexing Muscles Stock Photo - Download Image Now - Muscular Build, Swimming Pool, Teenage Boys - iStock06 fevereiro 2025 -
 irbsl.com.br at WI. IRBSL – Instituto Rio Branco06 fevereiro 2025
irbsl.com.br at WI. IRBSL – Instituto Rio Branco06 fevereiro 2025 -
إتحاد سيدي لخضر IRBSL06 fevereiro 2025
-
 modfx de tip top06 fevereiro 2025
modfx de tip top06 fevereiro 2025 -
 Filament + Resin 3D Print of Mass Effect Pistol : r/3Dprinting06 fevereiro 2025
Filament + Resin 3D Print of Mass Effect Pistol : r/3Dprinting06 fevereiro 2025 -
 انهزام يرهن الأمل - La Meskiana06 fevereiro 2025
انهزام يرهن الأمل - La Meskiana06 fevereiro 2025 -
 Imperial Charbroiler IRB-24 — Denver Cutlery, Inc.06 fevereiro 2025
Imperial Charbroiler IRB-24 — Denver Cutlery, Inc.06 fevereiro 2025
você pode gostar
-
 Volume 3 (Blu-ray & DVD) Sono Bisque Doll wa Koi wo suru Wiki06 fevereiro 2025
Volume 3 (Blu-ray & DVD) Sono Bisque Doll wa Koi wo suru Wiki06 fevereiro 2025 -
 Illaoi runes - Imgur06 fevereiro 2025
Illaoi runes - Imgur06 fevereiro 2025 -
 Raffle - 3D Printed Eevee - true to Pokédex scale – TreeHouseCustoms06 fevereiro 2025
Raffle - 3D Printed Eevee - true to Pokédex scale – TreeHouseCustoms06 fevereiro 2025 -
 ANIME DVD ~ MOB PSYCHO 100 SEASON 3 (ep 1-12 )-DHL EXPRESS06 fevereiro 2025
ANIME DVD ~ MOB PSYCHO 100 SEASON 3 (ep 1-12 )-DHL EXPRESS06 fevereiro 2025 -
![Roshar MTG Set Mechanic Showcase: BR Sacrifice [RoW] : r/Cosmere](https://preview.redd.it/roshar-mtg-set-mechanic-showcase-br-sacrifice-row-v0-tlz5mfvldbga1.jpg?width=640&crop=smart&auto=webp&s=1e9d735565a9431d1af5eee00b1b56f0544d9a2c) Roshar MTG Set Mechanic Showcase: BR Sacrifice [RoW] : r/Cosmere06 fevereiro 2025
Roshar MTG Set Mechanic Showcase: BR Sacrifice [RoW] : r/Cosmere06 fevereiro 2025 -
 NewPOP Editora on X: Fumetsu no Anata e (Uma Vida Imortal) está recheado de novidades este mês, hein! 😁 Uma delas é que o anime será dublado pela Crunchyroll. A dublagem está06 fevereiro 2025
NewPOP Editora on X: Fumetsu no Anata e (Uma Vida Imortal) está recheado de novidades este mês, hein! 😁 Uma delas é que o anime será dublado pela Crunchyroll. A dublagem está06 fevereiro 2025 -
 Mini Jogo Labirinto Animais da Quinta Sortido06 fevereiro 2025
Mini Jogo Labirinto Animais da Quinta Sortido06 fevereiro 2025 -
 Beast Tamer Manga Volume 6 Yuusha Party wo Tsuihou sareta Beast06 fevereiro 2025
Beast Tamer Manga Volume 6 Yuusha Party wo Tsuihou sareta Beast06 fevereiro 2025 -
 Jogo De Mesa Cirúrgico, De Emergência, Cirurgia De Bisturi Para06 fevereiro 2025
Jogo De Mesa Cirúrgico, De Emergência, Cirurgia De Bisturi Para06 fevereiro 2025 -
 Let Me Solo Her Legendary Creature - Human Tarnished Flash Let Me Solo Her can't be06 fevereiro 2025
Let Me Solo Her Legendary Creature - Human Tarnished Flash Let Me Solo Her can't be06 fevereiro 2025


