Statistics in Medicine — Reporting of Subgroup Analyses in
Por um escritor misterioso
Last updated 27 fevereiro 2025


A comprehensive analysis of the efficacy and safety of COVID-19 vaccines: Molecular Therapy

Breast Cancer, Reporting of older subgroups in registration breast cancer trials 2012–2021

Subgroup analysis in randomised controlled trials: importance, indications, and interpretation - The Lancet
Improving the Transparency of Prognosis Research: The Role of Reporting, Data Sharing, Registration, and Protocols

Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis - The Lancet

Understanding Clinical Data Analysis: Learning Statistical Principles from Published Clinical Research

What are descriptive statistics? - GCP-Service

Subgroup analyses. Deaths and tests for interaction by subgroup. HRs

Meta-Analysis Subgroups - Comprehensive Meta-Analysis

Statistical Analysis Plan: What is it & How to Develop it

Subgroup analysis in randomised controlled trials: importance, indications, and interpretation - The Lancet
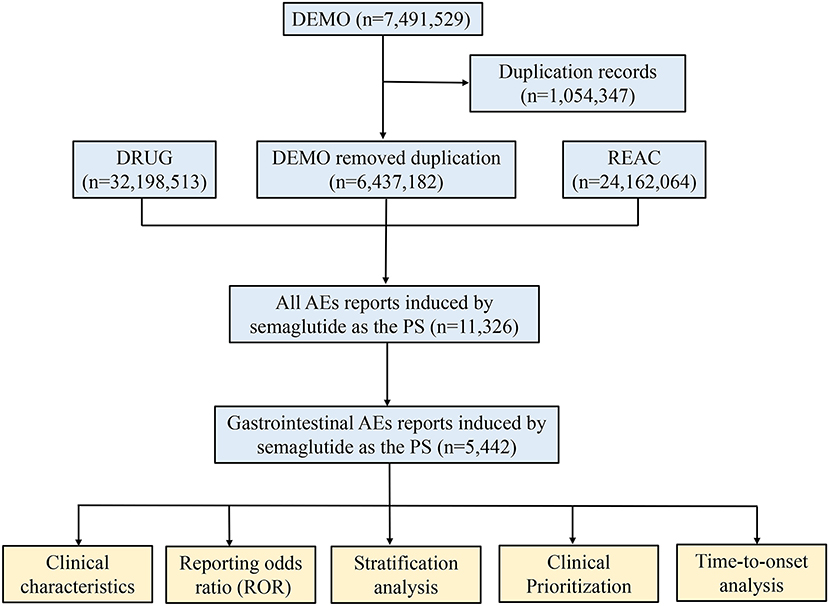
Frontiers Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system

Factors Associated With Racial and Ethnic Diversity Among Heart Failure Trial Participants: A Systematic Bibliometric Review
Recomendado para você
-
 How to Pronounce Analyses27 fevereiro 2025
How to Pronounce Analyses27 fevereiro 2025 -
 Hidden Consequences of Interim Analyses & Adaptive Trial Options27 fevereiro 2025
Hidden Consequences of Interim Analyses & Adaptive Trial Options27 fevereiro 2025 -
 Sample Analyses · SENAITE27 fevereiro 2025
Sample Analyses · SENAITE27 fevereiro 2025 -
 IRaMuTeQ analyses of COPM benefits. (a) Word cloud. (b) Similarity27 fevereiro 2025
IRaMuTeQ analyses of COPM benefits. (a) Word cloud. (b) Similarity27 fevereiro 2025 -
 Chapter 7 Subgroup Analyses27 fevereiro 2025
Chapter 7 Subgroup Analyses27 fevereiro 2025 -
 Announcing the Upcoming Release of the 2022 NBCOT Practice Analyses27 fevereiro 2025
Announcing the Upcoming Release of the 2022 NBCOT Practice Analyses27 fevereiro 2025 -
 Performing Statistical Analyses - SAS Video Portal27 fevereiro 2025
Performing Statistical Analyses - SAS Video Portal27 fevereiro 2025 -
 GAMLj: General Analyses for the Linear Model in Jamovi27 fevereiro 2025
GAMLj: General Analyses for the Linear Model in Jamovi27 fevereiro 2025 -
 Naval Analyses (@D__Mitch) / X27 fevereiro 2025
Naval Analyses (@D__Mitch) / X27 fevereiro 2025 -
 Rj Editor – Analyse your data with R in jamovi · jamovi27 fevereiro 2025
Rj Editor – Analyse your data with R in jamovi · jamovi27 fevereiro 2025
você pode gostar
-
 Goku SSJ God - SSGSS by GokuXdxdxdZ on DeviantArt27 fevereiro 2025
Goku SSJ God - SSGSS by GokuXdxdxdZ on DeviantArt27 fevereiro 2025 -
 Clipart cartoon of a tick check and cross x mark characters27 fevereiro 2025
Clipart cartoon of a tick check and cross x mark characters27 fevereiro 2025 -
 LIVE A LIVE HD-2D Remake Original Soundtrack, 下村陽子, ゲーム27 fevereiro 2025
LIVE A LIVE HD-2D Remake Original Soundtrack, 下村陽子, ゲーム27 fevereiro 2025 -
 DIY MINI Doll House Miniature DIY Dollhouse With Furnitures Wooden House Waiting Time Toys For Children Birthday Gift C00727 fevereiro 2025
DIY MINI Doll House Miniature DIY Dollhouse With Furnitures Wooden House Waiting Time Toys For Children Birthday Gift C00727 fevereiro 2025 -
 EXCLUSIVE PIKACHU is BACK! 😲 Kyoto Pokémon Center Oct 2023 FULL SHOP TOUR!! 🛍️27 fevereiro 2025
EXCLUSIVE PIKACHU is BACK! 😲 Kyoto Pokémon Center Oct 2023 FULL SHOP TOUR!! 🛍️27 fevereiro 2025 -
 Assistir The Kings Avatar Episodio 11 Online27 fevereiro 2025
Assistir The Kings Avatar Episodio 11 Online27 fevereiro 2025 -
Unitom - Revolution #revolution #tomógrafo #cascavel #unitom27 fevereiro 2025
-
 Brad Galli on X: Jamie Benn got ejected for this cross check to Mark Stone's head/neck. No place for this in hockey. / X27 fevereiro 2025
Brad Galli on X: Jamie Benn got ejected for this cross check to Mark Stone's head/neck. No place for this in hockey. / X27 fevereiro 2025 -
 Roar” (13-episode TV series, 1997). Heath Ledger as the p…27 fevereiro 2025
Roar” (13-episode TV series, 1997). Heath Ledger as the p…27 fevereiro 2025 -
Cute Curly Pigtails Aesthetic Hair Blue - Roblox27 fevereiro 2025
