CSPI asks FDA to ban powdered caffeine sold as a dietary supplement
Por um escritor misterioso
Last updated 13 fevereiro 2025

The Center for Science in the Public Interest (CSPI) has sent a petition to federal regulators seeking the ban of pure, powdered caffeine that is packaged and sold as a dietary supplement. Because of the product’s extreme potency, the possibility of accidental overdose poses a clear and present public health risk, the organization asserts.

FDA urged to ban concentrated caffeine powder
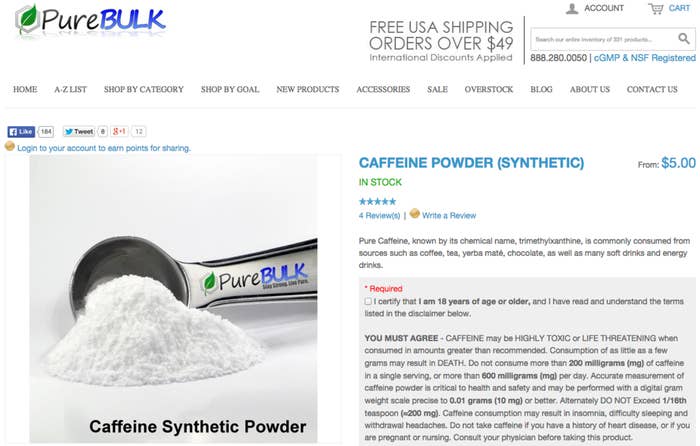
Powdered Caffeine Could Be Deadly, Government Warns

FDA Warns Against Concentrated Caffeine (Pure Powder & Liquids)

Latest Information About Meal Replacement Powders/Drinks: Product Reviews, Warnings, Recalls, & Clinical Updates from

New York, Other States Target Powdered Caffeine as FDA Ponders Possible Ban

FDA issues warning to powdered caffeine distributors

Does Prevagen Really Work?

PDF) Caffeine Khaled Hamlaoui
Full article: Abstracts

Just One Teaspoon of the World's Favorite Legal Drug Is Enough to Kill You

Caffeine - Food
Recomendado para você
-
 Bulk Supplements Ascorbic Acid (Vitamin C) Powder - 1kg for sale online13 fevereiro 2025
Bulk Supplements Ascorbic Acid (Vitamin C) Powder - 1kg for sale online13 fevereiro 2025 -
 Pre-Workout Bundle Pre-Workout Sampler13 fevereiro 2025
Pre-Workout Bundle Pre-Workout Sampler13 fevereiro 2025 -
Android Apps by BulkSupplements.com on Google Play13 fevereiro 2025
-
 BulkSupplements.com Creatine Monohydrate Capsules, 5000mg - Pre-Workout, Muscle-Building Supplements (300 Gel Caps - 43 Serv)13 fevereiro 2025
BulkSupplements.com Creatine Monohydrate Capsules, 5000mg - Pre-Workout, Muscle-Building Supplements (300 Gel Caps - 43 Serv)13 fevereiro 2025 -
 Inositol Powder - 4.5oz (Bulk Supplements) - Natures Medicinary13 fevereiro 2025
Inositol Powder - 4.5oz (Bulk Supplements) - Natures Medicinary13 fevereiro 2025 -
Who wants over 500+ pure bulk supplements with no fillers or additives, Regret Buying13 fevereiro 2025
-
 BulkSupplements.com Magnesium Glycinate Powder - Magnesium Bisglycinate, Magnesium Supplement, Magnesium Glycinate 400mg - Pure Magnesium Glycinate - 2200mg per Serving, 250g (8.8 oz) : Health & Household13 fevereiro 2025
BulkSupplements.com Magnesium Glycinate Powder - Magnesium Bisglycinate, Magnesium Supplement, Magnesium Glycinate 400mg - Pure Magnesium Glycinate - 2200mg per Serving, 250g (8.8 oz) : Health & Household13 fevereiro 2025 -
 Bulk Supplements Whey Review: HIGH Protein, NO Flavor13 fevereiro 2025
Bulk Supplements Whey Review: HIGH Protein, NO Flavor13 fevereiro 2025 -
 Bulk Supplement Manufacturer and Supplier13 fevereiro 2025
Bulk Supplement Manufacturer and Supplier13 fevereiro 2025 -
 Bulk Supplements13 fevereiro 2025
Bulk Supplements13 fevereiro 2025
você pode gostar
-
 Aniplay13 fevereiro 2025
Aniplay13 fevereiro 2025 -
 06 Desenhando Hinata Mestre Toy - Arts - Universo Geek13 fevereiro 2025
06 Desenhando Hinata Mestre Toy - Arts - Universo Geek13 fevereiro 2025 -
 Slay definition svg printable cut file13 fevereiro 2025
Slay definition svg printable cut file13 fevereiro 2025 -
 Finalmente tenemos los requisitos en PC de Detroit: Become Human13 fevereiro 2025
Finalmente tenemos los requisitos en PC de Detroit: Become Human13 fevereiro 2025 -
:quality(75)/cloudfront-us-east-1.images.arcpublishing.com/elcomercio/C5Z5Y6DDHNEMJKPFX6T55RASQM.png) Kimetsu no Yaiba Temporada 3 Episodio 10 online en Crunchyroll: fecha, hora y cómo ver Demon Slayer: Arco de la Aldea de los Herreros 3x010, Anime, nndaml, FAMA13 fevereiro 2025
Kimetsu no Yaiba Temporada 3 Episodio 10 online en Crunchyroll: fecha, hora y cómo ver Demon Slayer: Arco de la Aldea de los Herreros 3x010, Anime, nndaml, FAMA13 fevereiro 2025 -
 I can't believe it's the anniversary in 2023 the year that FNAF 313 fevereiro 2025
I can't believe it's the anniversary in 2023 the year that FNAF 313 fevereiro 2025 -
 Devil May Cry 5 Dante Rebellion 3D Printing Sword Cosplay Weapon Prop13 fevereiro 2025
Devil May Cry 5 Dante Rebellion 3D Printing Sword Cosplay Weapon Prop13 fevereiro 2025 -
 Livro: Minha Vez de Brilhar - Erin E. Moulton13 fevereiro 2025
Livro: Minha Vez de Brilhar - Erin E. Moulton13 fevereiro 2025 -
 O GTA 5 tem crossplay? – Tecnoblog13 fevereiro 2025
O GTA 5 tem crossplay? – Tecnoblog13 fevereiro 2025 -
 PlayStation 4 Slim 1TB Console13 fevereiro 2025
PlayStation 4 Slim 1TB Console13 fevereiro 2025
